Most of these criteria are consistent with the ones used in clinical trials from 20 to 15 years ago ( NINDS, ECASS-I, ECASS-II). However clinical practice hardly ever presents a patient ‘s profile that meets these criteria. That is justify according to our experience in several exclusion criteria, they should become relative and assessed individually.
After more than 2 decades of validity of these criteria we are aware that they get validated clinical trials must be strict to its success and its use in clinical practice, but we must perform a progressive exceptions of classical criteira about use rtPA, demonstrating their safety to another patient profile sometimes with increased morbidity.
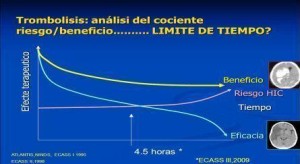
Below we reference the exclusion criteria of thrombolysis in acute stroke (ABSOLUTE contraindication, and in turn some RELATIVE since they have been applied in recent years to population subgroups not included in clinical trials). These criteria should be individualized RELATED then assessing risk-benefit and given with informed consent a compassionate use.
IMAGING OF EXCLUSION CRITERIA:
-Intracranial or subarachnoid hemorrhage, epidural, acute or chronic subdural.
-Demonstration of stroke larger than 1/3 of ACM (considered as such hypodense or similar ASPECTS <7)
-Presence of non-symptomatic intracranial tumor (meningioma despite being highly vascularized provide that not giants could be).
-Incidental presence of intracranial aneurysms or already treated.
– Other radiological findings that increase bleeding risk (MAV, cavernomas, telangiectasia). Or relative contraindication but unknown (intracranial bypass valves or devices whether recent or chronic).
EXCLUSION CLINICAL CRITERIA :
-Age. If> 80 is a relative contraindication.
-Rankin> 2. Rankin cases 3 or 4 would be a relative contraindication, but few general acute focal potential benefits it presents.
-More than 4.5 hours. Between 3 and 4.5h while not present any contraindication neuroimaging should be evaluated carefully.
-Recent treatment with antivitamin K oral anticoagulant therapy (acenocoumarol, warfarin) and have INR levels> 1.7 (It is not demonstrated the security of reverse anticoagulation clotting time to treat patietnts).
-Being treated with the new anticoagulants: apixaban, dabigatran, rivaroxaban, etc ..(no experience except no show ultimately takes 24-48 hours and normal clotting times)
-Thrombocytopenia (<100,000)
-Blood pressure > 185/105. Antihypertensive could allow your application if these limits BP get down. More than 2 bolus doses of antihipertensive (eg labetalol 100mg) with its corresponding “pause” in the infusion of rtPA makes its unpredictable response in our experience.
-Diabetes and prior stroke (symptomatic, but not in last 6 weeks). They were excluded from clinical trials (hemorrhagic risk, diabetic microaneurysms, etc.). Currently treatment is performed as a relative contraindication evaluating moderate clinical impairment and radiographic appearance.
-NIH clinical involvement with> 30 (supposed coma patient could be comatose patient with intubation, assess risk-benefit potential bleeding as multiple catheters, intubation, etc ..) and in any case as a relative contraindication and compassionate use.
-Endocarditis, acute pericarditis.
-Aortic arch dissection (important in any patient assessment peripheral pulse exam before rtPA infusion, especially if it was affected right cerebral hemisphere (involvement innominate artery).
-Severe hemodynamic instability. (Eg: multi-organ failure, septic shock .. 🙂
-Surgery in last 6 weeks. (Improvement guarantee 3 months from surgery)
– Stroke in last 6 weeks (check that there is no bleeding at that location).
-Gastrointestinal bleeding in 6 weeks and no active disease (guarantee 3 months prior).
-Lumbar punctures, deep arterial punction (pre 24h).Punctures between 48-72 would be a relative contraindication.
-Liver disease with impaired TP> 1.7 or liver failure
-Severe renal impairment is not a contraindication but should be assessed if necessary adjust doses given rtPA renal elimination.
-Other clinical aspects that physician judgment exceeded the risk to benefit from treatment. (abdominal aneurysms, acute pancreatitis, mucositis, head trauma* …)
* Bruising subgaleal could be a relative contraindication, assessing parenchymal detail in that region bruising and contralateral contusion and SAH. Especially headache presence and awareness level will be important signs to limit its use in these patients with cranial traumatism. If it is possible endovascular technique in these cases could be the choice. Important exam body injury bruising suggestive of fractures, bruises and others that may be complicated by the rtPA treatment or develop compartment syndrome